
Polymer Police by Andi Q. '25
Using spectroscopy to prove my mom wrong (except she was right and I was actually wrong oops)
Yesterday, my mom had some “silk” scarves shipped to me so I could bring them back home after graduation. She was very excited about them, telling me how happy all her friends would be when she gave them these “100% pure silk” scarves as gifts. However, I was skeptical of these scarves as soon as I unboxed them. The packaging was incredibly suspicious, with the scarves arriving in a box that literally said “CHEMICAL TEXTILE” in big bold letters. They also didn’t look or feel like other silk fabrics I was used to. Plus, my mom ordered them on the internet, where anyone can say whatever they want.
I was convinced that these scarves were made of some synthetic textile like polyester, while my mom was convinced that “of course it’s real silk, Andi, why would anyone on the internet lie to you”. Short of having her come to MIT and feel the fabric herself, there was little arguing I could do to change her mind. Clearly, I needed a scientific test to determine conclusively what material the scarves were made of.
Unfortunately, by far the most common scientific test for silk is to… burn the fabric01 Synthetic materials like polyester melt and smell like burning plastic, while silk burns slowly. . I couldn’t possibly do that! Damaging the scarves in any way was out of the question, given that they might be made of real silk and my mom would be very sad if I burned her merchandise. I also didn’t want to cause a fire alarm and have everyone in my dorm freeze to death in the Boston cold.
But then inspiration struck. MIT has tons of high-tech lab tools for characterizing materials. What if I just used one of those tools to find the polymer structure of the fabric? Conveniently, MIT’s Department of Materials Science and Engineering created the Breakerspace a year ago so that undergrads like me can use these tools for this exact purpose. Spectroscopy02 A technique that analyzes which wavelengths of light interact with a chemical, which can tell you things like which atoms the chemical is made of and how they are bonded together. doesn’t lie, so my mom would have to believe me after seeing the evidence!
Selecting the right tool to use was surprisingly difficult. Like the burn test, most of the tools would end up damaging the scarves in some way. For example, X-ray diffraction would involve grinding the fabric into a powder before irradiating it with X-rays, and Raman spectroscopy would involve shining a powerful laser onto the fabric (which would probably burn a hole into it). An electron microscope wouldn’t damage the fabric (although it would require me to cut out a small patch) and would tell me its chemical composition but wouldn’t reveal anything about the bonding structure, so that wouldn’t work either.
Luckily, the Breakerspace has the perfect tool for the job after all! Meet the Fourier-transform infrared (FTIR) spectrometer:

The FTIR spectrometer works by shining infrared light onto a material and then measuring how much the material absorbs/transmits the light at different wavelengths. The resulting spectrum can then be used to identify the material because sharp changes in absorbance/transmittance correspond to specific bonds between atoms. This happens because molecules are like balls (atoms) joined together with springs (bonds), and so the speed at which each ball can wiggle (and thus which wavelengths of light the wiggling absorbs) is dictated by how heavy the ball is and how many springs are attached to it.
For example, here are the FTIR spectra of silk and polyester:
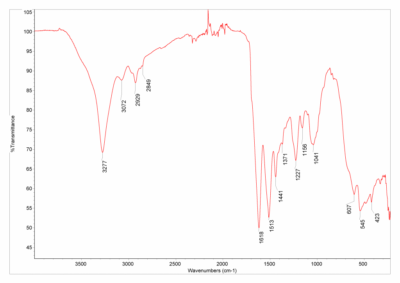
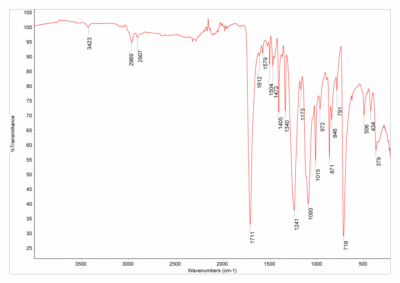
And here are their polymer structures:
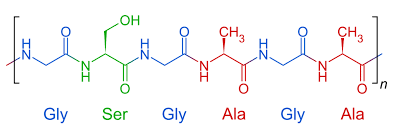
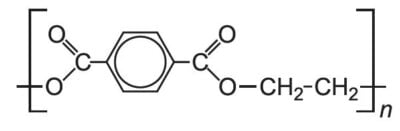
Silk’s FTIR spectrum (left) has a big dip that polyester’s spectrum (right) does not have. This dip is due to an oxygen-hydrogen bond stretching, which silk has (on the green part of the structure) but polyester does not have. Other differences between the two polymers (for example, silk has nitrogen while polyester has that hexagonal ring structure) contribute further to the differences between the spectra. Very cool stuff,03 There's some quantum sensing magic going on under the hood too, but I'm saving that for a future blog post. but I digress.
Anyway, the FTIR spectrometer was perfect for this little experiment for several important reasons:
- It only uses infrared light (and a tiny amount too), so there was almost zero risk of the tool damaging the fabric.
- There was almost no sample preparation required. I could quite literally drape the fabric over the tool and it would happily measure it. (See the image below.)
- It collects data very quickly. I think I had to wait less than a minute per sample.
- The data it collects is clean and consistent, which makes it very easy for me to identify the material.

Measuring a polyester t-shirt using the spectrometer.
So what did I find using the spectrometer?
Well…
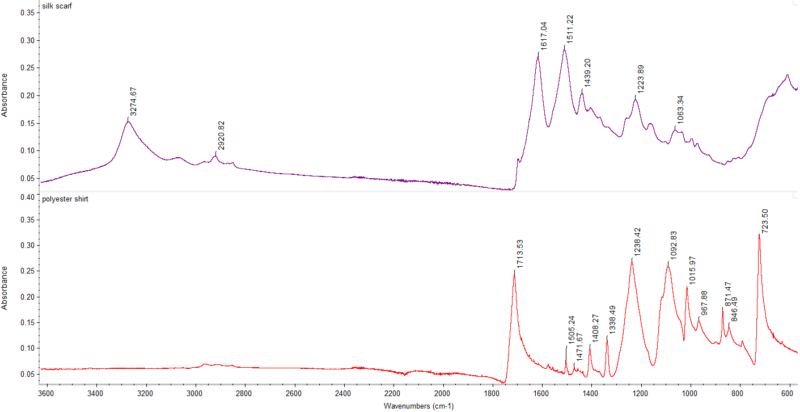
Absorbance FTIR spectra that I measured for the scarf (top) and a polyester t-shirt (bottom). These look like flipped versions of the spectra from above because absorbance is almost the opposite of transmittance here.
The scarf’s FTIR spectrum almost perfectly matched that of silk! For good measure, I also collected an FTIR spectrum of a 100% polyester shirt, which also matched the expected spectrum. Great news for my mom, because the scarves were genuine as she had hoped.
… so I was wrong. Oops. Not quite the mic-drop ending I was hoping for, but oh well. I guess the moral of this story is to trust everything on the internet, because why would anyone on the internet lie to you.
- Synthetic materials like polyester melt and smell like burning plastic, while silk burns slowly. back to text ↑
- A technique that analyzes which wavelengths of light interact with a chemical, which can tell you things like which atoms the chemical is made of and how they are bonded together. back to text ↑
- There's some quantum sensing magic going on under the hood too, but I'm saving that for a future blog post. back to text ↑